-
 Find in Members
Find in Members Find in Videos
Find in Videos Find in Channels
Find in Channels
This website uses cookies to ensure you get the best experience on our website.
To learn more about our privacy policy Click herePrivacy Preference
- Tags - #AAV Manufacturing #AAV Production #AAV Cell line
-
- Last updated June 30, 2022 0 comments, 1,028 views, 0 likes
- United States - Get Directions
More in Politics
Related Blogs
Archives
AAV Manufacturing Process from GenScript ProBio
Posted By GenScript ProBio
June 30, 2022
Body
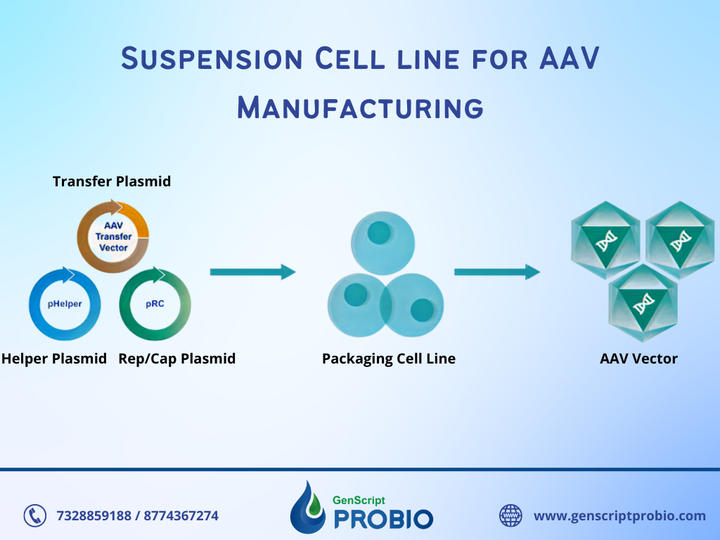 AAV manufacturing is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs.
AAV manufacturing is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs. Map
-
Locations on MyWorldGo
Location Information
- Location: United States - Get Directions
- Formatted Address: United States
- Country: United States







Comments