-
 Encontrar enMiembros
Encontrar enMiembros Encontrar enVideos
Encontrar enVideos Encontrar enCanales
Encontrar enCanales
This website uses cookies to ensure you get the best experience on our website.
To learn more about our privacy policy haga clic aquíPreferencia de privacidad
- Etiquetas - #AAV Manufacturing #AAV Production #AAV Cell line
-
- Última actualización 30 de junio de 2022 0 comentarios, 1.010 vistas, 0 likes
- United States - Obtener las direcciones
More in Politics
Related Blogs
Archivo
AAV Manufacturing Process from GenScript ProBio
Publicado por GenScript ProBio
30 de junio de 2022
Cuerpo
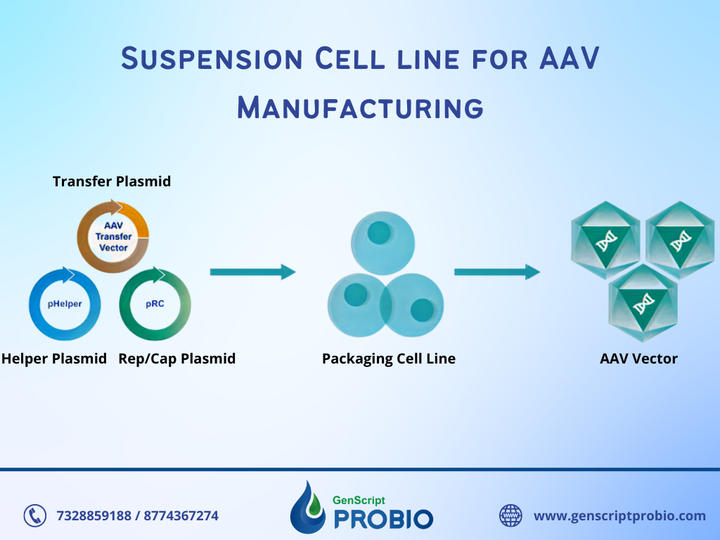 AAV manufacturing is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs.
AAV manufacturing is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs. Mapa
-
Ubicaciones en MyWorldGo
Información sobre la ubicación
- Ubicación: United States - Obtener las direcciones
- Dirección formateada: United States
- País: United States







Comentarios