-
 Find in Members
Find in Members Find in Videos
Find in Videos Find in Channels
Find in Channels
This website uses cookies to ensure you get the best experience on our website.
To learn more about our privacy policy Click herePrivacy Preference
- Tags - #EU Authorised Representative
-
- Last updated April 23, 2023 0 comments, 212 views, 0 likes
More from I3c Global
More in Politics
Related Blogs
What is CE Marking? For Medical Devices, It Means A Whole Lot:
Body
A blog that explains how to become regulated and certified for your device.
When it comes to certifying a new device for the European market, there are a number of regulations you'll have to consider. The regulations most directly affecting your device include the Medical Device Directive (MDD),
In Vitro Diagnostic Device Directive (IVDD), and the In-vivo Medical Device Directive (IVDMD). Essentially, these directives dictate what type of CE marking that you'll need to apply to your medical device product in order to sell it in Europe.
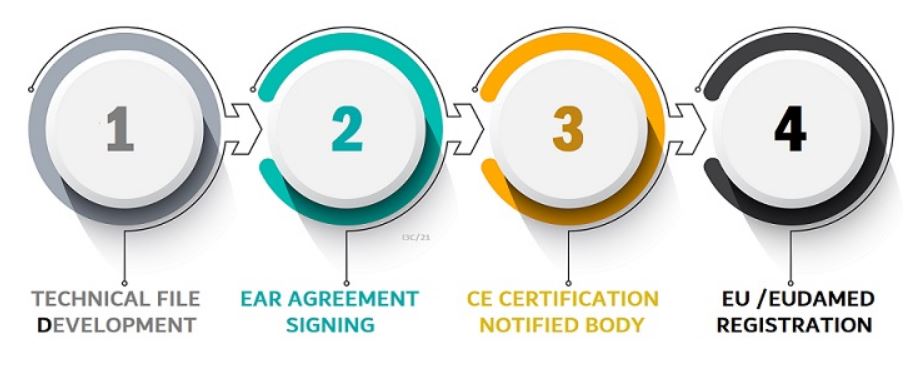
What does a medical device manufacturer do when they want to get certified in Europe?
Manufacturers must register their products with Eudamed-MED (the European Medicines Agency). To do this, they will need to complete an application form and provide a list of all their intended marketing authorizations. Once registered, manufacturers receive a Unique Device Identification (UDI) number for each product. The UDI number can be used as part of any marketing activity in the EU Authorised Representative such as advertising or labeling.
Manufacturers must have their product assessed by a notified body that has been granted approval by an EU Member State. Notified bodies assess whether or not products comply with MDR and other relevant EU legislation (e.g., electrical safety, electromagnetic compatibility). They also perform conformity assessments audits on manufacturers
Do all medical devices need to be CE Marked?
No, not all medical devices require CE marking. Only Class Ia, Ib and IIa devices need to be CE marked under Directive 93/42/EEC. Class III devices do not require CE marking because they are not intended for use in humans or animals but may be used if they are specifically authorized by another legislation such as Directive 2011/65/EU as amended by Directive 2012/19/EU (medical device directive).
If you're looking for CE Marking services, don't hesitate to contact us, we are one of the professional China examiners and consultants in Canada, if you want more information about CE Marking service and certification visit our website www.i3cglobal.uk









Comments