Gene Therapy for Cystic Fibrosis
Body
What is cystic fibrosis?
Cystic fibrosis (CF) is a rare genetic disorder that affects the lungs, digestive system, and other organs. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a protein that regulates chloride ions transport across cell membranes. These mutations cause the CFTR protein to be either absent or not function properly, leading to the buildup of thick, sticky mucus in the lungs, pancreas, and other organs.
Treatment of Cystic Fibrosis
CF is a chronic and progressive disease with no known cure, but there are several treatments available that can help manage the symptoms and improve the quality of life for people with the disease. These treatments include antibiotics to treat lung infections, airway clearance techniques to clear mucus from the lungs, nutritional support, and medications that target specific mutations in the CFTR gene.
Multiple clinical trials are currently underway to test new therapies for CF, including gene therapy and drugs targeting specific mutations in the CFTR gene. In addition, researchers are exploring new methods, such as CRISPR genome editing, to treat CF at the gene level. As research continues, people hope that new treatment methods will continue to improve the lives of CF patients and ultimately cure this devastating disease.
Cystic Fibrosis Genetic Therapies
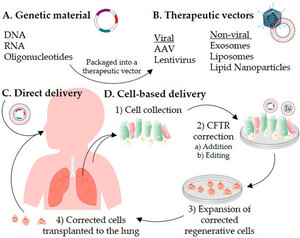
Since the discovery of the CFTR gene in 1989, scientists have been working on correcting CF mutations. Gene therapy is a process where a new, correct version of the CFTR gene is placed into a person's cells. The presence of the correct copies allows cells to produce normal CFTR proteins. Advances in gene therapy in the past decade have accelerated progress toward CF cure.
Two types of gene therapy have the potential to treat CF, including non-integrated and integrated gene therapy. In non-integrated gene therapy, a single DNA fragment containing the correct copy of the CFTR gene is delivered to human cells, but it is not permanently integrated into the genome. Non-viral vectors have been developed for delivering the CFTR gene. A major advantage of these non-integrated gene delivery methods is that it does not disrupt the rest of the genome. However, because it is not permanent, gene therapy effects may last only a few weeks or months.
In integrative gene therapy, a piece of DNA containing the correct CFTR gene is delivered to a human cell and becomes a permanent part of the genome. One approach to integrative gene therapy is to use viral vectors, such as retroviruses, adenovirus (Ad), adeno-associated viruses (AAV), and helper-dependent adenoviruses (Hd-Ad), to deliver the corrected CFTR gene. While this approach has shown promise in preclinical studies, permanent gene modification raises concerns about long-term effects.
Current Gene Therapy Clinical Trials in Cystic Fibrosis
There are currently several ongoing clinical trials testing different gene therapy approaches for CF. One of the most promising gene therapies in development is KB407. This is a replication-defective, non-integrated herpes simplex virus type 1 (HSV-1)-derived vector engineered to deliver functional full-length human CFTR to CF patients' airways via nebulization. Currently, the FDA has approved Krystal Biotech's new drug research application, allowing the company to initiate phase I clinical trials of KB407 in CF patients.
Another promising gene therapy in development is 4D-710, an experimental aerosol gene therapy developed by 4D Molecular Therapeutics (4DMT). 4D-710 is designed to deliver an artificial version of the CFTR gene through the 4D-A101 AAV vector, providing instructions for making a working version of the CFTR protein. By delivering the gene to lung cells, the therapy is designed to increase CFTR protein function, normalize mucus production, and alleviate CF symptoms. 4DMT is in a Phase 1/2 clinical trial testing 4D-710 in approximately 21 CF patients.
In addition, nanoparticle-encapsulated mRNA delivery techniques are becoming more advanced and have rapidly expanded gene therapy. A phase 1/2 clinical trial is currently underway to test the delivery of an mRNA encoding the full-length CFTR to the lungs of CF patients using lipid nanoparticles. This drug (MRT5005) could potentially enable in vivo lung gene editing.
Currently, large-animal CF models are used to validate novel therapies and identify pathophysiologically relevant target cell types for effective CF gene therapy. The success of gene therapy will hopefully define a path for other complex rare diseases.
Reference
- Allan, K. M.; et al. Treatment of cystic fibrosis: from gene-to cell-based therapies. Frontiers in pharmacology, 2021, 12: 639475.









Comments