Gene Therapy for Duchenne Muscular Dystrophy
Corps
What is Duchenne Muscular Dystrophy?
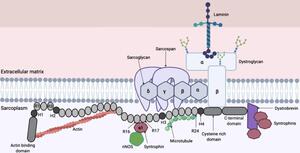
Duchenne muscular dystrophy (DMD) is a fatal neuromuscular disease caused by mutations in the dystrophin gene on the X chromosome. The mutation results in a lack of dystrophin in patients' muscles, a protein that keeps muscle cells intact and, when deficient, leads to progressive muscle degeneration and atrophy. DMD also affects the heart and respiratory muscles, leading to life-threatening complications later in the disease.
DMD is the most prevalent and severe form of myotonic dystrophy. Patients typically lose the ability to walk independently around age 12 and die at age 19 due to heart failure or postoperative infection.
Duchenne Muscular Dystrophy Treatment Options
While there is currently no cure for DMD, there are several treatment options that can help manage the symptoms and slow the progression of the disease.
RNA exon skipping for Duchenne Muscular Dystrophy
Exon-skipping therapy is a new treatment option for DMD and works by using synthetic molecules called antisense oligonucleotides (ASOs) to skip over the faulty sections of the dystrophin gene, allowing for the production of a shortened but functional version of the dystrophin protein. Two exon-skipping therapies have been approved by the FDA for DMD: Eteplirsen and Golodirsen. While these therapies have shown promise in clinical trials, they are not effective for all DMD patients, and their long-term efficacy is still being evaluated.
Gene Therapy for Duchenne Muscular Dystrophy
Gene therapy is a promising new treatment aimed at slowing or stopping DMD progression. This therapy involves using selected segments of the dystrophin gene to create a smaller, potentially functional version, known as a microdystrophin protein. This transgene-encoded microdystrophin is then delivered to muscle cells via a single infusion of an adeno-associated virus (AAV) vector. Microdystrophin is designed to stabilize muscle, which is a function typically performed by the essential dystrophin protein missing in individuals with DMD.
Recently, the FDA Advisory Committee concluded that the benefits of Sarepta Therapeutics' gene therapy SRP-9001 for DMD outweigh its risks, supporting accelerated approval of the therapy. This is the first FDA-approved gene therapy for DMD, offering significant hope for DMD patients.
Current Gene Therapy Clinical Trials in Duchenne Muscular Dystrophy
Although gene therapy does not completely cure DMD or restore muscle cells lost, it shows promise for stabilizing the progression of symptoms and improving strength and endurance in some individuals with DMD who usually become weaker. Several clinical trials of AAV-delivered microdystrophin proteins for DMD are currently underway, including from Pfizer, Sarepta, Genethon, and Solid Biosciences.
PF-06939926 is an intravenous AAV gene therapy developed by Pfizer that uses rAAV9 to deliver a shortened myotonic dystrophy protein gene for therapeutic purposes. It was previously granted orphan drug status and Rare Pediatric Disease Designation (RPDD) by the FDA. The drug candidate completed the first patient dose in a Phase 3 trial. The clinical trial is currently in progress.
SGT-001 is an AAV9-mediated gene therapy developed by Solid Biosciences. The therapy works by delivering a copy of the gene encoding microdystrophin to muscle cells to express a functional substitute for microdystrophin in muscle tissue and stabilize the necessary proteins. SGT-001 has been granted RPDD and orphan drug status by the FDA. SGT-001's safety and efficacy are currently being evaluated in the IGNITE-DMD Phase I/II clinical trial.
CRD-TMH-001, developed by Cure Rare Disease, is designed to deliver a CRISPR gene editing system directly into the body via a large number of viruses to restart the backup anti-myasthenic protein gene in patients. The IND application for CRD-TMH-001 is currently approved by the FDA. It is also the first CRISPR gene editing therapy approved to enter clinical trials for DMD.
At present, although several DMD drugs have been approved for marketing, they are still far from meeting clinical needs. Emerging therapies in development for this disease, including exon skipping therapy, gene therapy, cell therapy, and gene editing, are now entering the clinic. This promises safer options for patients.
Reference
- Elangkovan, N.; Dickson, G. Gene therapy for Duchenne muscular dystrophy. Journal of Neuromuscular Diseases, 2021, 8(s2): S303-S316.











commentaires