Research on the Biological Effects of GLP-2
Body
GLP-2 (glucagon-likepeptide-2) was discovered and named by the Lilly Laboratory in the United States when cloning the proglucagon gene (PG). In 1996, Drucker et al. discovered that the active ingredient in proglucagon-derived peptide (PGDP) that promotes intestinal mucosal growth is GLP-2. Its effect is stronger than other growth factors that have been discovered, and its growth-promoting effect is organ-specific (limited to the stomach and intestine), GLP-2 began to be valued and studied. Subsequent animal experiments and human studies have shown that the main function of GLP-2 is to stimulate the proliferation of intestinal mucosal crypt cells and inhibit their apoptosis, thereby promoting the growth of intestinal mucosa and regeneration and repair after injury.GLP-2 can also inhibit the secretion of gastric acid and motility, increase the blood supply of intestine, improve the barrier function of the intestine, and promote the absorption of nutrients in the intestine.
GLP-2 and Glucagon-Derived Peptides
For mammals, GLP-2 uses the proglucagon gene (PG) as a template and is transcribed in pancreatic A cells, intestinal L cells, hypothalamus, brainstem and other central neurons, and is expressed and translated. It is processed into a polypeptide containing 33 amino acids with a molecular weight of 3900. The proglucagon gene includes 6 exons and 5 introns, and its mRNA is translated into a single-chain precursor protein containing 160 amino acids. It is tissue-specific and is activated by prohormone convertase ( PCs) undergo post-translational processing to generate a series of proglucagon-derived peptides (PGDP) with different biological activities. PGDP includes glucagon (glucagon), proglucagon fragment (MPGF), and glucagon-related pancreatic polypeptide (GRPP) secreted by pancreatic A cells, as well as enteroglucagon and oxyntomodulin in the intestine and brain, glucagon-like peptide-1 (GLP-1), glucagon-like peptide 2 (GLP-2), intermediate peptide 1 (IP-1) and intermediate peptide 2 (IP-2), etc. The main form of GLP-2 in the blood circulation is complete GLP-2, and there is also partial GLP-2 formed after the first two amino acid residues at the amino terminus are hydrolyzed by diacyl peptidase IV (DPPIV).This form of GLP-2 is inactive.
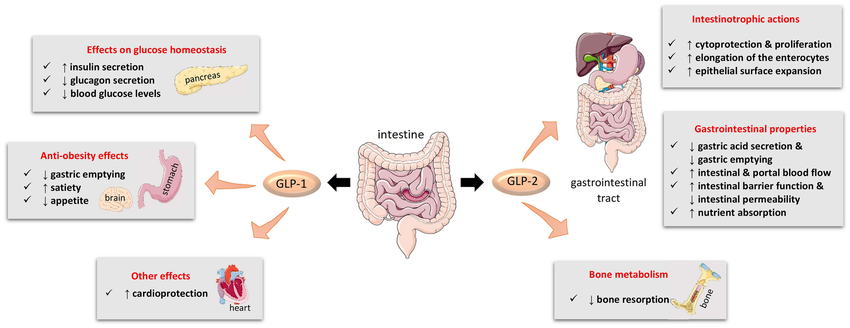
Figure 1.Main biological actions of GLP-1 and GLP-2.
Physiological Functions and Pharmacological Effects of GLP-2
Different from other polypeptide growth factors, GLP2 is an intestinal epithelial-specific growth factor with intestinal specificity and stronger growth-promoting effect. Researchers used subcutaneous injection to compare the growth-promoting effects of GLP-2 EGF (epidemal growth factor), IGF-1 (in-sulin-growth factor-1) and GH (growth homone) on normal intestinal mucosa in mice., found that GLP2 has the most obvious effect. In the study on the effect of intestinal adaptation in short-intestine rats, it was found that the GLP-2 group had a significantly higher effect on increasing the weight of the jejunal intestine and the height of villi than the GH group and the KGF (keratinocyte growth factor) group.In addition, research data shows that GLP-2 mainly promotes the proliferation of intestinal epithelial cells and has no effect on the proliferation of other important organ tissues such as heart, liver, and kidney cells. No pathological changes have been seen in these tissues after long-term application of GLP-2.
GLP-2 Promotes Normal Small Intestinal Growth
GLP-2 can promote the increase in the weight and length of the stomach and small intestine of newborn rats during the lactation period. GLP2RmRNA is expressed at high levels in the stomach and small intestine of rat embryos and neonatal stages. The concentration of GLP2 in plasma increases before the birth of fetal pigs. It is significantly increased and maintained at a high level throughout the entire period of breast milk nutrition, which suggests that GLP2 may play an important role in the development and maturation of the gastrointestinal tract.
GLP2 Promotes Recovery of Damaged Intestinal Mucosa
A phase I clinical trial using teduglutide (a GLP-2 analogue resistant to DPPIV degradation) to treat patients with ulcerative colitis showed that the remission rate of ulcerative colitis in the treatment group was higher than that of the placebo group, and the therapeutic effect was dose-dependent.
GLP-2 Affects Gastric Acid Secretion and Gastrointestinal Motility
GLP2 inhibits human gastric acid secretion caused by sham feeding. Sham feeding causes gastric acid secretion to increase approximately 5 times. This increase is reduced by 65% in the GLP-2 perfusion group compared with the normal saline perfusion group, indicating that GLP-2 is a powerful inhibitory factor for human gastric acid secretion. As an inhibitory factor, animal experiments have also proven that GLP-2 can inhibit gastric emptying in pigs.
GLP-2 Increases Intestinal Blood Supply
In newborn piglets fed total parenteral nutrition, administration of GLP-2 can increase portal blood flow by 25%, and this reaction is dependent on nitric oxide. Interesting, GLP-2 increases portal blood flow only in the small intestine, because it was found that the increase in hemoglobin content and the expression of GLP-2 receptors were mainly concentrated in the small intestine.
GLP2 and Feeding
Studies have found that the plasma level of GLP-2 in healthy people increases significantly after eating, but physiological levels of GLP2 have no significant effect on appetite and energy intake.
GLP-2 Inhibits Bone Resorption
Porosis may be the result of bone loss due to an imbalance in bone remodeling, which causes bone resorption to exceed bone formation. Common treatments inhibit bone resorption by reducing osteoclast number, activity, and longevity, but subsequently bone formation is also inhibited. The study found that exogenous GLP-2 caused a sharp and sustained reduction in bone resorption but had no effect on immediate bone formation. It can be seen that GLP-2 can affect the balance of bone remodeling, and the tilt of this balance toward osteogenesis may eventually lead to an increase in bone mass and bone strength, thus providing a new model for the treatment of osteoporosis. GLP-2 treatment reduces bone resorption but does not affect bone formation, and may have a more positive impact on bone health than therapies that reduce bone resorption and bone formation at the same time.
Post-receptor Signal Transduction Mechanism of GLP-2
A large number of studies have shown that GLP2 regulates the proliferation of intestinal epithelial cells and inhibits their apoptosis by acting on the GLP2 receptor (GLP-2R), thereby protecting intestinal cells. The human GLP2R gene is located on chromosome 17p13.3. Cloning of human and rat cDNA-encoded GLP2R shows that it is a member of the G protein-coupled receptor superfamily, with 7 transmembrane domains, similar to GLP-1 glucagon and GIP (Glucose-dependent insulinotropic polypeptide) receptors have a high degree of homology. A series of studies using immunohistochemistry, in situ hybridization, confocal laser microscopy, RT-PCR and Westem blot analysis separately and/or in combination showed that GLP-2R is expressed in intestinal epithelial cells, gastric epithelial cells, intestinal intrinsic neurons. It is expressed in enteroendocrine cells, intestinal, submucosal myofibroblasts, vagal afferent fibers, cerebral cortex, cerebellum, hypothalamus, amygdala, hippocampus, dentate gyrus and lungs. The distribution density of GLP2R in the gastrointestinal tract is in the jejunum, duodenum, ileum, colon, and stomach. However, the signal transduction mechanism of GLP2R is still unclear, mainly due to the lack of cell models for studying GLP-2R.









Comments